Lesson 2: Classification of Materials
In this section we will look at the classification of materials from the perspective of what needs to be seen and done to determine the appropriate classification. We will talk about a variety of different types of materials. There are a variety of ways of making those classifications and of representing those classifications. You may already be familiar with mixtures, solutions, pure substances, compounds, and elements and be able to distinguish between them by considering their physical properties. If so, you may want to skip ahead to the short study check for this topic and verify that you already know what will be covered in this section. This section will take you step-by-step through a procedure to classify materials into the categories listed above.
Using a Flowchart | Step 1: Visual Inspection | Step 2: Try Phase Changes
Step 3: Try Chemical Decomposition | Conclusion | Study Check
Using a Flowchart
| The method of classification used here employs a flow chart approach. A series of questions or tests is applied to the materials to be classified. The answers or results of these questions or tests determines what category the material is placed into. It is kind of like the game "twenty questions," but we will need only three questions or tests. Each of these questions is described in the following pages. In the flow chart outline shown here each diamond-shaped box represents a question or test of some kind and each rectangular box represents a category into which the material "being questioned" is placed. |  |
Step 1: Visual Inspection
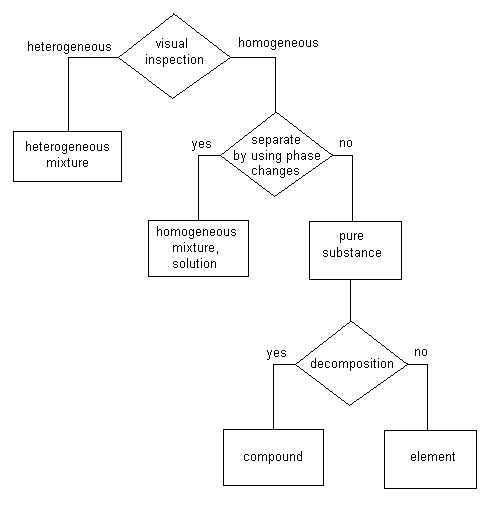
In this scheme the first test is a visual inspection. Look at the material and determine whether it is heterogeneous or homogeneous.
"Heterogeneous" means that you can see more than one material present. If you can see that a material is heterogeneous, it is classified as a "heterogeneous mixture." Sometimes this is shortened simply to "mixture." For our purposes this is not a good idea just yet. If you have a mixture of salt and pepper, for example, you can look at it and see both the salt and the pepper. It is a heterogeneous mixture. You can see that there is more than one component to that material. Cork is another example of a heterogeneous mixture. Most bricks are. Dirt is. These are all heterogeneous mixtures. |
 |
If visual inspection shows that a material is homogeneous, additional questions will have to be asked before we can put that material into a category.
Step 2: Try Phase Changes
The next question to ask is whether the material can be separated into components by using phase change methods such as distillation. Materials which are separated into components when they go through distillation or sublimation or some other phase change are classified as "homogeneous mixtures" or "solutions." Materials which are not broken up into components or separated into components by going through phase change operations are classified as "pure substances."
|
The distillation of water can serve as an example. If the water has something dissolved in it (making it a solution), distillation will evaporate the water and leave the other component behind. Seeing this, we know that there were two (or more) components in the original material and thus it must have been a mixture. On the other hand, if nothing was dissolved in the water, distillation would not separate anything from the water. Seeing this, we know that the water was pure and was not a mixture. |
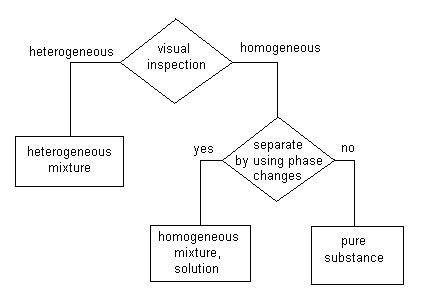 |
Note that we now have two categories of mixtures, heterogeneous mixtures and homogeneous mixtures. Because homogeneous mixtures are usually called solutions, heterogeneous mixtures are sometimes simply called mixtures. Keep in mind that a material which is referred to as a mixture might be homogeneous or heterogeneous.
Step 3: Try Chemical Decomposition
As mentioned previously, materials which are not broken up into components or separated into components by going through phase change operations are considered to be pure substances. It turns out that pure substances can be of two types. The two types of pure substances are called compounds and elements. Both compounds and elements are pure substances.
|
The test for whether a pure substance is a compound or an element is to find out whether it can be decomposed. If a pure substance can be chemically decomposed it is classified as a compound. If a pure substance cannot be chemically decomposed it is classified as an element. Water is an example of a compound. It is an example of a compound because it is a pure substance that can be decomposed into components. In the electrolysis exercise in this lesson water is separated into its components. When an electric current is passed through water, it is able to separate the hydrogen from the oxygen. Because water is a pure substance that can be decomposed, we classify it as a compound. Elements, on the other hand, are pure substances which cannot be decomposed by electrolysis or any other kind of chemical reaction. |
 |
Conclusion
So, there you have the different types of categories or classifications of materials with which you need to be familiar for this lesson. A material can be classified as a heterogeneous mixture, a homogeneous mixture or solution, or a pure substance. If it is a pure substance, then it can be further classified as either a compound or an element.
I will have quite a bit more to say about elements later on in this lesson and in the course, but at the moment just let me say that over the years and over the centuries, people have tried to figure out what were the simplest possible things, the things which could not be further broken down, and called them elements. The number of things considered to be elements has increased considerably over the years. At one point air, earth, fire, and water were considered to be the elements. Air and earth are now known to be mixtures, fire is a form of energy released in a chemical reaction, and water is a compound. Other materials have supplanted these four as elements. Currently, we have about 90 naturally occurring elements and another dozen or so have been artificially created.
Study Check
Answer the following questions on classification and check your answers below.
1. A homogeneous liquid which can be separated into components by distillation is
classified as a/an:
a. pure substance
b. mixture
c. solution
d. element
e. compound
2. A homogeneous liquid which cannot be separated into components by distillation but
can be decomposed by electrolysis is classified as a/an:
a. pure substance
b. mixture
c. solution
d. element
e. compound
Answers to Study Check on Classification of Materials
1. A homogeneous liquid which can be separated into components by distillation is
classified as a/an:
a. pure substance
b. mixture
*c. solution
d. element
e. compound
2. A homogeneous liquid which cannot be separated into components by distillation but
can be decomposed by electrolysis is classified as a/an:
a. pure substance
b. mixture
c. solution
d. element
*e. compound