Lesson 2: Combinations of Atoms
One of the important reasons for talking about atoms is because the atoms combine with one another. That was an important part of the reason that Dalton came up with his atomic theory. He wanted to explain why compounds had fixed composition. But, as you will see, atoms combine in more ways than Dalton imagined.
The diagrams shown here (and in Example 8 of your workbook) represent Dalton's concept of forming compounds by the combination of individual atoms of different elements. The first shows how hydrogen and oxygen atoms can combine to make what he called composite atoms or compound atoms of the compound water. Today we would call them molecules. The compound water is formed, he thought, by one hydrogen atom combining with one oxygen atom to form one molecule of water. (There is a serious problem with that statement that I will get to in just a moment.) |
|
||
The second shows how the compound sodium chloride is formed. One atom of sodium combines with one atom of chlorine to form one unit (call it a molecule for now if you want) of sodium chloride. |
|
Essentially, that was Dalton's concept of a compound. The value of this approach is that it explains constant composition. The mechanism of how those atoms hook together is not yet explained, but the idea that they do hook together gives some sense to the elements combining in fixed ratios.
There are, however, short-comings. One is that water is not composed of one atom each of hydrogen and oxygen. Another short-coming is that it doesn't address the different types of compounds and the different ways in which atoms can bond to one another. I chose these particular examples, water and salt (sodium chloride), because they represent radically different ways of combining elements, of combining atoms. We will deal with those different ways of combining atoms quite a bit later in this course when we talk about bonding, but I did want to point out that short-coming now. Another problem is that Dalton's method ignores the combination of like atoms. It doesn't say anything about two atoms of oxygen combining together, and I think Dalton denied that that was even a possibility. We know now, of course, that it is more than just a possibility. It does, in fact, happen.
In this section, we will take a closer look at molecules, formulas and the various types of formulas with which you need to be familiar.
Molecules | Formulas | Types of Formulas
Molecules
While some chemists were exploring the weight relationships of the elements, others were looking at the volume relationship in the chemical reaction of gases. When you use electrolysis to decompose water hydrogen gas and oxygen gas are the products. By volume, twice as much hydrogen as oxygen is generated -- a 2:1 ratio by volume. Also, the reaction can be reversed; hydrogen and oxygen combine in a simple 2:1 ratio by volume to make water. Simple relationships like that catch people's attention. Other gaseous chemical reactions also involved simple whole number volume ratios. It seems that gases always reacted in simple whole number ratios by volume. This relationship is called the Law of Combining Volumes. The French chemist Gay-Lussac came up with it in 1808.
One implication of this was that water contained twice as much hydrogen as oxygen, and therefore water molecules consisted of two hydrogen atoms combined with one oxygen atom, rather than one of each, as Dalton had proposed. How to decide which was correct, however, was not obvious.
An Italian chemist by the name of Amadeo Avogadro came up with an idea, a hypothesis, in 1811. It is now called Avogadro's Law. After studying the reactions of some gaseous elements, he proposed that equal volumes of gases (under the same conditions) contain the same number of molecules. He also proposed that a molecule was a combination of atoms--a little pile of atoms. One of the reactions he had apparently been studying was the formation of water from hydrogen and oxygen. By carrying out the reaction above the boiling point of water, the water that was formed remained in the gaseous state. So both of the reactants and the product as well were all gases. What he noticed was that two volumes of hydrogen combined with one volume of oxygen to form two volumes of water. (By "a volume" I mean whatever size measurement of volume you want to use.) It took twice as much hydrogen as oxygen and the volume of water formed was equal to the volume of the hydrogen that you started with. It's as if the oxygen moved in with the hydrogen. As I mentioned before, this was our first clue that water consisted of two parts hydrogen to one part oxygen. This was not explained by Dalton's version of atomic theory and so Dalton just considered it to be of no particular consequence. |
|
Avogadro, however, thought it was very important and developed the concept that I will try to illustrate here (and in example 9 of your workbook). The boxes are all supposed to be the same size and represent equal volumes of gases and they contain equal numbers of molecules. I will use just one molecule in each. The hard part was to make the idea work. However, once you know the solution, it's not hard at all. |
|||||||||||
We start with the idea that each molecule of water contains two hydrogen atoms and one oxygen atom. |
|
||||||||||
The two oxygen atoms in the two water molecules come from one oxygen molecule that contains two oxygen atoms. |
|
||||||||||
Each hydrogen molecule also contains two atoms providing the four hydrogen atoms necessary for the two water molecules. |
|
||||||||||
Notice that all this is derived from the assumption that water molecules contain two hydrogen atoms with one oxygen atom and Avogadro's hypothesis that equal volumes of gases contain equal numbers of molecules. |
Avogadro's new concept contained the idea that molecules of hydrogen react with molecules of oxygen to form molecules of water and that each molecule of hydrogen and each molecule of oxygen contains two atoms rather than just one. The importance of this was quite apparent to Avogadro, but it took several decades before the importance of his hypothesis was brought to the front and used to convince people of what was going on in these gaseous reactions.
Molecules vs. Compounds
Note that there is a very important distinction between compounds and molecules. A comparison of Dalton's ideas and Avogadro's ideas points this out. You may at times hear that elements are made up of atoms and compounds are made up of molecules, but it turns out that is not true on several counts. In water, two hydrogen atoms are hooked to an oxygen atom to form both a molecule and a compound. However, when two hydrogen atoms are hooked together they form a molecule, but they do not form a compound. Both atoms are the same element, not two different elements. The same is true with oxygen. Two oxygen atoms hooked together form a molecule but they do not form a compound. Thus, it is possible to have molecules of an element.
Sodium chloride brings up a related issue. Although it is a compound, it is not a molecular compound. The way that the sodium and chlorine are hooked together involves a different mechanism and one that does not result in the formation of sodium chloride molecules. Instead, they make a crystalline network. Sodium chloride is a network compound rather than a molecular compound. That becomes important later in the course when we deal with bonding.
Although it might be convenient to equate molecules and compounds, they are not the same thing. There are materials which are molecular but are not compounds, like hydrogen and oxygen. There are materials which are compounds but are not molecular, like sodium chloride. There are materials which are neither compounds nor molecular like magnesium. There are also materials, like water, which are both molecular and compounds. So you must treat those two concepts--molecules and compounds--separately. What they do have in common with one another is that they both represent combinations of atoms, but they are different kinds of combinations.
Review
Elements consist of tiny particles called atoms. Atoms of the same element can combine with one another to form molecules, but they are not compounds. Atoms of different elements can also combine with one another to form molecules, and these combinations are compounds. It is also possible for atoms to combine with one another in ways that do not form molecules. Instead, they can form vast arrays or networks of atoms sometimes called crystal lattices.
A compound is a combination of atoms of different elements (with a fixed ratio). Water is an example of a molecular compound; sodium chloride is an example of a nonmolecular compound.
A molecule is a combination of atoms bonded in a certain way to form a small collection or cluster of atoms that are all hooked together. The atoms in the molecule might all be the same (element) or they might be different (compound).
Formulas
Now, since we know that atoms can combine with one another we have to have some way of representing this. The way that we show combinations of atoms on paper is to write down combinations of symbols for those atoms. These combinations of symbols are called formulas. Much like we can make words by combining letters, we can make formulas by combining symbols. (Ex. 10 in your workbook.)
Dalton's formula for water would be HO. We write down HO together, with no space in between them, and that would represent one atom of hydrogen combined with one atom of oxygen. (Again, that is not the correct formula for water.)
Avogadro's formula for water would be H2O. H2O simply means that there are two atoms of hydrogen bonded to one oxygen atom, a ratio of two hydrogen atoms for every one oxygen atom. The subscript follows the symbol of the element to which it refers.
The way that you would represent sodium chloride is to write down the symbol Na followed by the symbol Cl with no space between them. That represents sodium chloride--a combination of sodium and chlorine in a 1:1 ratio by atoms.
The formula for molecular hydrogen would be written as H2. The 2 subscript immediately following the H means that there are two hydrogen atoms combined with one another. The formula for molecular oxygen is O2. Again, the subscript 2 shows that there are 2 of those oxygen atoms bonded together. Note that the formulas for these elements is different from the symbols for these elements because the formulas represent the molecules of the elements and the symbols represent the atoms of these elements.
Here is the formula for some kind of a sugar molecule. C6H12O6 represents 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
In the formula Fe(NO3)3 you have to contend with parentheses. The 3 at the end of the formula--the one that is outside the parentheses--means that we have 3 of everything inside the parentheses. So there are 3 NO3's which would be a total of 3 nitrogen atoms and nine oxygen atoms, along with the 1 iron atom. Notice that the last 3 doesn't apply to the iron. It only applies to what's inside the parentheses. The 3 inside the parentheses applies only to the oxygen.
You can also write down the formula of the compound if you are given the information about how many atoms are in it. For example, if I told you that a particular compound had 1 sulfur atom for every 3 oxygen atoms, you should be able to write down the formula SO3. Or if I told you that in a particular molecule there were 2 nitrogen atoms and 5 oxygen atoms, you should be able to write down the formula N2O5.
Practice Interpreting Formulas
To give you a little more practice on this, why don't you try your hand at figuring out how many calcium atoms, how many phosphorus atoms, and how many oxygen atoms are represented by the formula Ca2(PO4)3 (in example 10d). Take a moment to figure that out, the answer follows.
Answer
You should have come up with 2 calcium atoms, 3 phosphorus atoms, and 12 oxygen atoms. Hopefully, you didn't have any problem figuring that out.
Types of Formulas
There are several types of formulas: molecular, structural, and empirical. Most of the formulas we have been dealing with have been molecular formulas. Different formulas are useful in different situations; let's look at what each kind of formula can tell us.
Molecular formulas apply to any molecular material. A molecular formula tells you the actual number of each kind of atom within that molecule. H2 shows that two hydrogen atoms are contained in the molecule. Similarly, O2 shows that two oxygen atoms are contained in the molecule. H2O shows that a water molecule contains two hydrogen atoms and one oxygen atom. Hydrogen peroxide has the formula H2O2, meaning that its molecules each contain two hydrogen atoms and two oxygen atoms.
Structural formulas also apply to molecular materials. They not only tell you how many of each kind of atom there is but which atoms are bonded to one another. The structural formula tells you something about the arrangement of atoms within the molecule. Water, for example, can be written as H-O-H, showing that the oxygen atom is in the middle and the two hydrogen atoms are bonded to it. The structural formula for hydrogen peroxide can be written as H-O-O-H.
Empirical formulas apply to any type of compound, whether it consists of molecules or not. Empirical formulas are formulas that are derived from experimental data. (Empirical means experimental.) All that is really shown about the compound in an empirical formula is the ratio of atoms. The very nature of how you go about calculating those formulas only allows you to get the simplest ratio.
Here are some examples. H2O is both an empirical and a molecular formula. The simplest ratio of hydrogen atoms to oxygen atoms is 2:1. Therefore, H2O is an empirical formula. However, if you can isolate individual molecules of water and figure how many atoms there really are in each one, it turns out there are 2 hydrogen and 1 oxygen atoms bonded together in the cluster that forms the molecule. So H2O is a molecular formula as well. In hydrogen peroxide, the simplest ratio of hydrogen atoms to oxygen atoms is 1:1, one hydrogen atom for every oxygen atom. So the empirical formula is HO. That is different from the molecular formula, which is H2O2.
A molecule like glucose shows how exaggerated the differences can be. The structural formula shows how the 24 atoms are hooked together. The molecular formula shows that each molecule contains 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms. The empirical formula simply shows that the ratio of atoms is 1:2:1.
| Structural Formula | Molecular Formula | Empirical Formula |
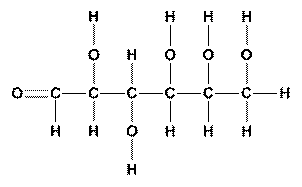 |
C6H12O6 | CH2O |
So these are three different kinds of formulas. The empirical formula gives you the simplest ratio. The molecular formula applies only to molecular materials and it gives you the actual number of each kind of atom that's contained in a molecule. The structural formula shows which atoms are bonded to which. We'll spend more time with empirical formulas later in this lesson. (Structural formulas will be dealt with in more detail later in the course.)