Lesson 6: Early Work
The development of the periodic table began in the 1800s as chemists started to recognize similarities in the properties of various elements and place them into familes. Döbereiner started with a few family groups each containing three elements. Newlands put the elements of those familes in repeating groups called octaves (as with musical notes). Meyer noted additional repating properties. Mendeleev put it all together into what has become the periodic table of the elements. The sections on this page give you some information about what they did.
Döbereiner | Newlands | Meyer | Mendeleev
Döbereiner
Some of the first elemental similarities were noted by a German chemist named Döbereiner in 1829. |
|||||||||||||||||||||||||
His observations began with bromine which had just been discovered. He noticed that the properties of bromine were similar to chlorine and iodine. Not only were they similar but various properties of bromine, including the atomic weight, fell midway between the properties of chlorine and iodine. Not only were there similarities in the properties but also there was a pattern or trend within the group of regularly increasing atomic weights. |
|
||||||||||||||||||||||||
He noticed a couple other groups of elements with patterns like this. They were calcium, barium, and strontium; also sulfur, selenium, and tellurium. He described these groups as being triads, groups of three elements that had similar properties. Not much was made of this because the triads only covered one-sixth of the known elements. Most chemists of the time considered them to be inconsequential coincidence. |
|||||||||||||||||||||||||
Newlands
Somewhat later, about 1864, a chemist by the name of Newlands came up with what he called the law of octaves. This idea was a bit more developed than Döbereiner's triads. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Newlands arranged the known elements by atomic weights. In doing so, he noticed some recurring patterns, and the patterns were such that if he broke up his list of elements into groups of seven (starting a new row with the eighth element), the first element in each of those groups were similar to one another. So was the second element in each group and the third and so on. There was a certain pattern in the properties of elements that became even more apparent as time went on. In the 1860s quite a bit of new information developed. In no small part this was due to the ideas of Avogadro begin championed by Stanislao Cannizarro at the First International Chemical Congress in Karlsruhe, Germany in 1860. |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Meyer
In 1870, Lothar Meyer, a German chemist, made a chart that plotted atomic volumes
against atomic weight. That plot is shown here using data from the "Periodic Chart of
the Atoms" (1979 Edition, Sargent-Welch Scientific Company).
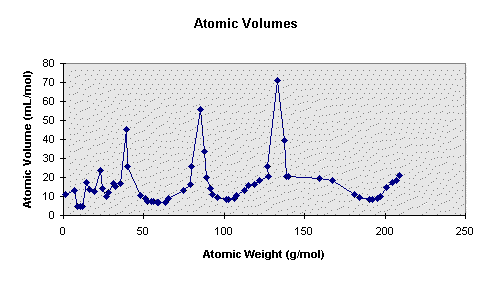 He
measured the volume of one atomic weight's worth of each element--that is, one mole--and
figured that since the number of atoms in each amount was the same, the volumes measured
must represent the relative volumes of the individual atoms. By plotting those volumes
against the atomic weights you can see that there is a recurring pattern--sort of like
waves with the crests and the troughs, or hills and valleys. We can start with the element
at the top of each one of those peaks. They are lithium, sodium, potassium, rubidium, and
cesium. Following each of those there is a repeating pattern. An important observation
that Meyer made was the change in length of that repeating pattern. Unlike Newslands’
octaves, these groups were not all the same length. Hydrogen was sort of a group all by
itself, lithium through fluorine was another group, sodium through chlorine was another,
potassium through bromine, and so on. Notice that there are small groups at the beginning
and then larger groups afterwards. In summary, there is repeating periodicity of the
atomic volume, but the periods changed in size. The first period is one element in
length--hydrogen. The second and third periods are seven in length. The fourth and fifth
periods are seventeen elements in length. Subsequent to Meyer's work the inert gases were discovered, so we now have one more element in each period--making 2, 8, 8, 18, 18.
He
measured the volume of one atomic weight's worth of each element--that is, one mole--and
figured that since the number of atoms in each amount was the same, the volumes measured
must represent the relative volumes of the individual atoms. By plotting those volumes
against the atomic weights you can see that there is a recurring pattern--sort of like
waves with the crests and the troughs, or hills and valleys. We can start with the element
at the top of each one of those peaks. They are lithium, sodium, potassium, rubidium, and
cesium. Following each of those there is a repeating pattern. An important observation
that Meyer made was the change in length of that repeating pattern. Unlike Newslands’
octaves, these groups were not all the same length. Hydrogen was sort of a group all by
itself, lithium through fluorine was another group, sodium through chlorine was another,
potassium through bromine, and so on. Notice that there are small groups at the beginning
and then larger groups afterwards. In summary, there is repeating periodicity of the
atomic volume, but the periods changed in size. The first period is one element in
length--hydrogen. The second and third periods are seven in length. The fourth and fifth
periods are seventeen elements in length. Subsequent to Meyer's work the inert gases were discovered, so we now have one more element in each period--making 2, 8, 8, 18, 18.
Mendeleev
The most famous work that was done in developing the periodic table was done by a Russian chemist, Dmitri Mendeleev. Mendeleev developed his periodic table in 1869. It was a table, not a graph. It was a two-dimensional arrangement in which elements having similar properties were placed adjacent to one another. It took several forms as it developed.
He began by lining up the elements in order of their atomic weights, just as Meyer was doing at about the same time. His first published tables listed the elements vertically.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Later, a horizontal table was published. Mendeleev incorporated three very important features.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Still later, when more was known about atomic structure and electron configurations, we found out that those electron configurations could also be related to the shape of the periodic table. That provided more evidence for the structure of the periodic table being a legitimate arrangement. That also indicated that the chemical properties were related to the electron configuration.
The importance of the electron configurations, combined with Meyer’s idea of different lengths of periods and the subgroups within the families, led to a change in the shape of the periodic table, giving us what we now call the Long Form of the Periodic Table. That is the form that is in most common use today. To see the change from the short form to the long form imagine the elements whose symbols are written to the left in each box of the short form being pulled en masse to the left and those written to the right in each box being pulled en masse to the right.
Long Form of the Periodic Table
| H | He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | La | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac | § | |||||||||||||||
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| § | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||