Lesson 8: Covalent Bonding Basics
In this section we consider the second of our three types of strong atomic bonds. Covalent bonding is what we call the strong attraction that holds together the atoms of nonmetallic elements. Covalent bonding is associated with a great variety of materials. It is found in elements and in compounds. It is found in networks and in molecules. It is also found within the polyatomic ions. Essentially it is found in any material in which nonmetallic atoms are bonded together.
Linus Pauling, after whom our science buildings are named, was foremost among those chemists who worked on the problem of covalent bonding and how it works. |
In this section, we will look first at the basics of covalent bonding, specifically what happens to the electrons of the atoms involved in the bonds.
Sharing Electrons | The Octet Rule | Forming Molecules
Sharing Electrons
In covalent bonding, both atoms are trying to attract electrons--the same electrons. Thus, the electrons are shared tightly between the atoms. The force of attraction that each atom exerts on the shared electrons is what holds the two atoms together.
The shared electrons of a covalent bond can be represented using Lewis diagrams. The bond can be emphasized by using a line to "hold the atoms together." The electron dots that are not involved in the bond are sometimes shown and sometimes not shown. (We will be showing those "non-bonding pairs" in our Lewis diagrams in this course.) |
|
Variations
Although the basic idea is that a covalent bond is the sharing of electrons between atoms, there are many ways this can happen. Usually, the electrons are shared between two atoms, but they can be shared by several atoms. |
|
Usually two electrons are shared, but there can be more. Two electrons shared between two atoms is called a single bond. Four electrons shared between two atoms is called a double bond. Six electrons shared between two atoms is called a triple bond. |
|
Usually each atom provides one electron, but not necessarily. If both of the shared electrons come from one atom, it is called a coordinate covalent bond. |
|
On top of all that, the atoms might share the electrons equally or unequally. If both atoms have the same attraction for the shared electrons, it is called a nonpolar covalent bond. If one atom has a stronger attraction for the shared electrons, it is called a polar covalent bond. |
|
You will see all of these variations as we look at some of the elements and compounds and networks and molecules that have covalent bonding.
Hybrid Orbitals
When atoms share electrons the s, p, d and f orbitals of the atoms change shape and become directed into the regions between atoms. Perhaps I should say they become pointed at the other atoms so that the electrons will be in the right place to be shared with the other atoms.This process is called hybridization of the orbitals. We won't dwell on the hybridization process in this lesson. We will, however, lump together all of the valence electrons and not worry about whether they came from s, p, d or f atomic orbitals. |
|
For nonmetals this generally means we are only concerned about the valence electrons, the s and p electrons in the highest energy level. The diagram here shows atomic (s and p) orbitals on the left and hybrid orbitals made from them on the right. |
|
Atomic Lewis Diagrams
You will be learning to draw Lewis diagrams for molecules in this lesson. For review and practice, I would like you to determine the electron dot diagrams (Lewis diagrams) for these elements which we will be working with during the next few pages. (These are also listed in exercise 1 in your workbook.)
![]()
Answers
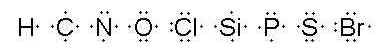
Let me go over these with you briefly. Hydrogen has one valence electron so its electron dot diagram has one dot. Carbon has four. Nitrogen has five. Oxygen has six. Chlorine has seven. Silicon, like carbon, has four valence electrons. Phosphorus, like nitrogen, has five. Sulfur, like oxygen, has six. Bromine has seven.
It is not particularly important exactly where those dots go in the diagram, although generally they are put to the left, to the right, above and below. (You should not have more than two electrons per side.) It is usually convenient to do the electron dot diagrams in pencil so that if you need to move the electrons for one reason or another, such as bonding, it will be easy to do so.
The Octet Rule
If we accept the idea that nonmetals like to gain electrons, a logical next question is, "How many?" The simple answer is, "However many more will fit in that energy level." Because of the way that electrons fill up energy levels, a slightly better answer is "however many more electrons are needed to fill the s and p sublevels of that energy level." Usually that means "enough to make a total of eight in the outer shell." This principle is called the octet rule.
The octet rule says that atoms tend to gain, lose or share electrons so as to have eight electrons in their outer electron shell. It is a very useful rule but you should also know that there are many bonding situations where it does not apply. As you learn to use the octet rule, also learn to recognize situations where it does not apply and disregard it in those situations.
Practice
Take a moment to apply the octet rule to the elements for which you just drew Lewis diagrams (exercise 1) to determine how many electrons each of these atoms would like to gain. Keep in mind that hydrogen cannot possibly fit eight electrons into its outer shell. Check the answers that follow when you are done.
Answers
| Electrons to be gained by ... | ||||||||
| H | C | N | O | Cl | Si | P | S | Br |
| 1 | 4 | 3 | 2 | 1 | 4 | 3 | 2 | 1 |
Hydrogen would like to gain one more electron. Notice that we start off with an exception to the octet rule because there is only room for a maximum of two electrons in the first energy level. Carbon would like to gain four more electrons. Nitrogen has five valence electrons so it would like to gain three more. Oxygen has six valence electrons and thus would like to gain two more. Chlorine has seven valence electrons and would like to gain one more. Silicon has four valence electrons and would like to gain four more. Phosphorus has five valence electrons and would like to gain three more. Sulfur has six and would like to gain two more. Bromine has seven and would like to gain one more valence electron according to the octet rule.
Forming Molecules
As you will see, covalent bonding usually results in a limited number of atoms being bonded together. These clusters of atoms are called molecules. An important distinction between ionic and covalent bonding is that ionic bonding results in network materials and covalent bonding usually results in molecular materials. There are some exceptions. In a few situations covalent bonding results in network materials. Those cases will be pointed out when we get to them.
Molecules are the clusters of atoms that we get when a limited number of atoms are covalently bonded together. That "limited number" may be a small number, as will be the case with the molecules we deal with in this lesson. It may be a few dozen as in many of the organic compounds. It may be hundreds or thousands of atoms as in many of the biochemical compounds.