Lesson 7: Buffers
A buffer solution makes use of Le Chatelier’s principle to prevent its pH from changing very much when acid or base is added.
Exercise 17 in the lab will show you how the pH changes when an acid or a base is added first to plain water, and then to a buffered solution. The difference can be quite dramatic. In fact, it is only because your bodily fluids, especially your blood, are heavily buffered, that you can survive eating a handful of pickles with no ill effects.
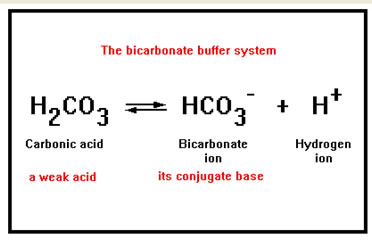
A buffered solution is one that contains relatively high concentrations of both a weak acid and its conjugate base. A simplified version of the equilibrium that buffers your blood is show here as an example.
This bicarbonate buffer system is what keeps the pH of your blood constant. However, carbonic acid, H2CO3, is unstable and decomposes to form carbon dioxide and water. Some of the carbon dioxide stays dissolved in the blood and some of it is released into the air in your lungs.
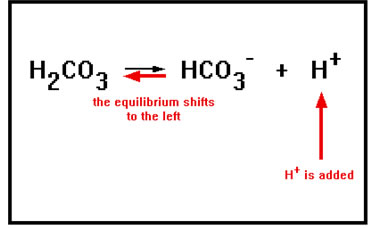
If some process releases acid [H+] into the blood, this equilibrium responds by shifting to the left as long as there is HCO3- present for the H+ to react with, thus removing the excess H+ ions. Thus, to protect against added acid, the buffer solution must have a relatively high concentration base, HCO3- , in solution. |
 |
If a process releases base into the blood, which would react with and lower the concentration of the H+, the equilibrium shifts to the right as long as there is H2CO3, thus replacing the lost H+ ions. Thus, to protect against added base, the buffer solution must have a relatively high concentration of the weak acid as well. |
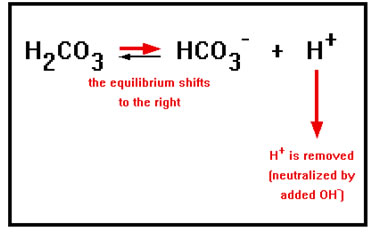 |
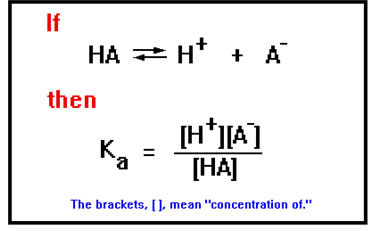
The equation on your screen describes the behavior of buffer systems in general. The value of the acid dissociation constant, Ka, depends on what acid HA represents.
This equation also correctly predicts Le Chatelier’s Principle. Suppose, for example, that the system is at equilibrium (the equation is satisfied) and then some H+ is added. With the extra H+ ions, the value of [H+][A-]/[HA] is too high. To return it to its correct value, [H+] and [A-] must decrease and [HA] must increase, that is, the equilibrium must shift to the left, just as Le Chatelier’s principle predicts.
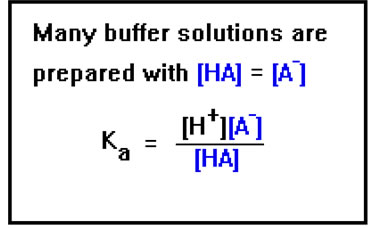
To maximize the buffering capacity against both added acid and base, the concentrations of A- and HA must both be high and, in fact, approximately equal.
The exact values of [A-] and [HA] depend on what pH the buffer is trying to maintain. There is a range of about 2 pH units within which each conjugate acid-base pair can function effectively as a buffer. This means that the two concentrations can usually be no more than a factor of ten different from one another.
But if the concentrations of A- and HA are equal, they cancel from the equation, and the H+ concentration will therefore be the same as Ka, the acid dissociation constant. If [A-] and [HA] are not the same, it is a simple matter to solve the equation for [H+] and plug in the values of Ka, [A-], and [HA]. The pH is then just the negative logarithm of this value. |
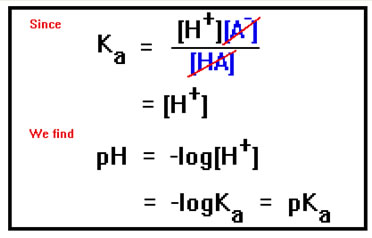 |
Thus, knowing the value of the Ka for a weak acid allows you to calculate the [H+] for a buffer made from that acid and its conjugate base. If the two concentrations are the same, the calculation is especially easy.
|
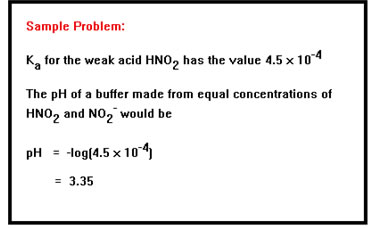 |
Use the data in Example 8 in your workbook to calculate the pH of three different buffer systems: the HF/F- system, the HC2H3O2/C2H3O2- system, and the NH4+/NH3 system. In each case assume that the concentrations of acid and conjugate base are the same. Check your answers below.
Answers:
HF/F- system: pH = 3.19
HC2H3O2/C2H3O2- system: pH = 4.74
NH4+/NH3 system: pH = 9.25
Notice that none of these buffer systems have a neutral pH. The purpose of a buffer is not to maintain a neutral pH; the purpose of a buffer is to maintain whatever pH the system is at, whether it be acidic, basic, or neutral.
This is the end of Lesson 7. Be sure to complete the lab exercise on buffers at the end of the workbook and turn in the problem set as well.
Lesson 8 deals with two important concepts that were introduced in this lesson, equilibrium and reaction rate (also called kinetics), and it also takes up the topic of the energy changes associated with chemical reactions. These are themes that are central to understanding chemical reactions.