Lesson 7: Hydrolysis
A hydrolysis reaction is one in which a water molecule breaks apart into H+, a proton, and OH-, a hydroxide ion.
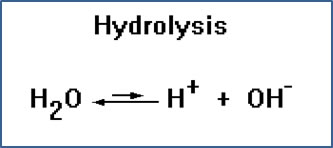
The word “hydrolysis” comes from the Greek works “hydro” meaning water, and “lysis” meaning to break apart.
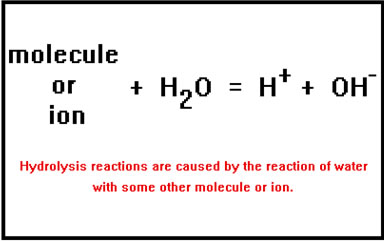
Although water molecules do occasionally break apart on their own, some other molecule or ion must be present to cause a significant number of them to do so.
 |
In pure water at room temperature, only about one out of every 2 x 10-9 water molecule is separated into H+ and OH- ions at any one time. This corresponds to a concentration of H+ (and OH-) of about 1.0 x 10-7 M. The negative log of this number, 7, is the pH of neutral solution. The solution is neutral not because the pH is seven, but because the concentrations of H+ and OH- are the same. At different temperatures, pure, neutral water has slightly different pH’s. |
If the molecule or ion that causes the hydrolysis reaction to occur then the bonds to the H+ ion that forms, the solutions will be left with an excess of OH- ions and will therefore be basic.
F- + H2O
OH- + HF
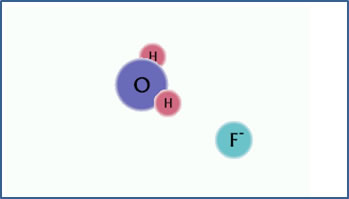 |
A fluoride ion in aqueous solution. |
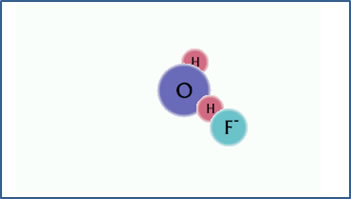 |
The fluoride has a strong attraction for hydrogen ions. |
 |
After the hydrolysis reaction, the solution contains free hydroxide ion and HF molecules. The solution is basic. |
Since the H+ ion is positively charged, a negative ion or a neutral molecule with a strongly electronegative atom is required for this to happen. In this example, the F- ion has a strong attraction for H+ ions, leaving behind an excess of free OH- ions in the solution.
On the other hand, if the molecule or ion that causes the hydrolysis to occur then bonds to the OH- ion that forms, the solution will be left with an excess of H+ ions and will therefore be acidic.
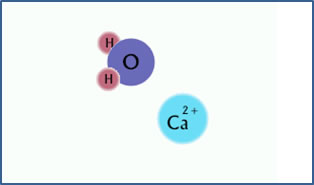
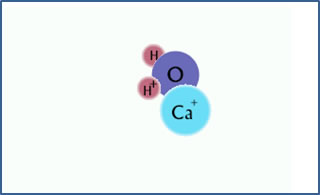
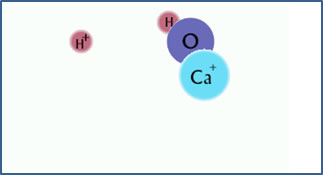
Ca2+ + H2O
CaOH+ + H+
|
Calcium ion in solution. |
|
The calcium ion has a strong attraction for hydroxide ions. |
|
After the hydrolysis reaction, there is free hydrogen ion and CaOH+. The solution is acidic. |
By the same token, to bond to the OH- ion requires a positive ion, or a neutral molecule with a strongly electropositive atom. In this example, the Ca2+ ion could actually bond to two hydroxide ions. If the concentration of Ca2+ is great enough, this will, indeed occur, and solid Ca(OH)2 will precipitate from solution, turning it cloudy.
It’s also possible that what causes the hydrolysis reaction to occur will bond to both the H+ ion and the OH- ions that form. In this case, the solution will be neutral, since neither H+ nor OH- ions are left in solution.
 |
For this to happen requires a molecule that has a strongly electronegative atom and also a strongly electropositive atom, or, as in this example, an ionic compound whose negative ion binds to the H+ and whose positive ion binds to OH-. |
In Chemistry 106 you will encounter a series of reactions in which more complex molecules are split in two and during which water molecules are also split. Because water molecules are split too, these reactions are said to involve hydrolysis of the more complex molecule.
You can think of water as consisting of a H+ ion bonded to a OH- ion. Water is a stable compound because these two ions have a strong attraction for one another.
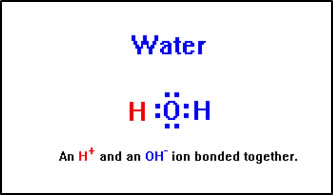 |
This is a bit of an oversimplification. It implies that the two hydrogen atoms in water are somehow different and, of course, they are not. What is true is that the hydogen-oxygen bonds are quite polar, so that the two hydrogens have a partial positive charge and the oxygen has a partial negative charge. It is therefore relatively easy to split one of the hydrogens off of the water molecule leaving the shared electrons behind, resulting in a H+ and an OH- ion. |
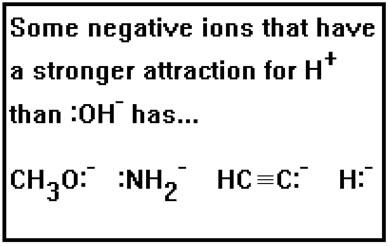
But there are other negative ions that have an even stronger attraction for the H+ ion than the OH- ion does. If one of these should be dissolved in water, it will have a strong tendency to “take” the hydrogen ions away from the water molecules and leave the OH- ions behind.
A neutral molecule that you have seen with a strong attraction for H+ ions is ammonia, NH3. Ammonia does not have as strong an attraction for H+ ions as the hydroxide ion does, but it’s strong enough to cause a significant number of water molecules to hydrolyze, leaving behind a significant number of hydroxide ions.
By the Brønsted-Lowry definition, these ions are all strong bases precisely because of their strong tendency to accept a proton.
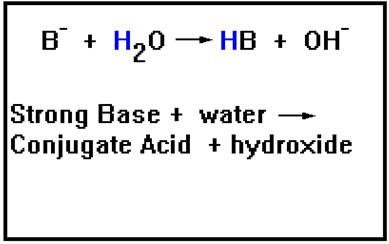 |
And they are bases by the Arrhenius definition as well beause they cause hydroxide ions to form in water.
|
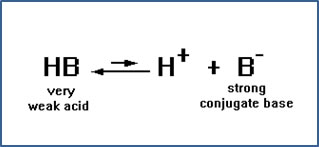
If the ion B- has a strong attraction for the proton, then the HB molecule will have a very weak tendency to give the proton up. In other words, HB will be a very weak acid.
In fact, the conjugate acids of the ions we have been looking at, CH3OH (the conjugate acid of CH3O-), NH3 (the conjugate acid of NH2-), HCCH (the conjugate acid of HCC-), and H2 (the conjugate acid of H-) are so weakly acidic, they are not considered acids at all.
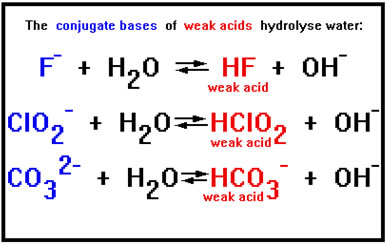
But a negative ion does not have to have a stronger attraction for H+ ions than OH- ions do in order to cause a significant number of water molecules to hydrolyze. The conjugate base of any weak acid will cause enough water molecules to hydrolyze to turn a solution basic. Most negative ions fit this description. In fact, our list of strong acids includes only six members: HCl, HBr, HI, HNO3, H2SO4, and HClO4. Their conjugate bases are Cl-, Br-, NO3-, HSO4-, and ClO4-. These very weak conjugate bases do not cause hydrolysis – their attraction for H+ ions is simply not great enough. All the other negative ions we will encounter form solutions of varying degrees of base strength when they are dissolved in water. |
 |
These ions can be added to water as part of a soluble ionic compound, usually a sodium or potassium salt. For example, when sodium fluoride is dissolved in water, the solution becomes basic because the fluoride ion causes a hydrolysis reaction. The second reaction occurs because HF is a weak acid. It is also necessary, of course, for the NaF to be soluble, or the second reaction never gets a chance to occur. However, virtually all sodium and potassium salts are soluble in water. |
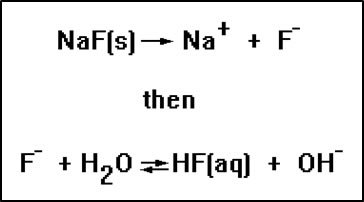 |
|
|
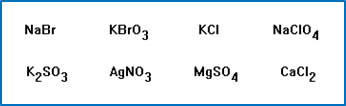
Select the compounds that you think will cause basic hydrolysis.
|
Once you have completed this exercise, see if you can write the hydrolysis reactions that will occur. Here’s an example: KBrO3 will cause hydrolysis because HBrO3 is a weak acid. The hydrolysis reaction that will occur is
|
Here are the answers you should have determined:
KBrO3 |
The hydrolysis reaction that will occur is
|
K2SO3 |
|
Positive ions can also cause hydrolysis reactions. But positive ions do so by binding to the OH- rather than the H+. As a result, positive ions cause the solution to turn acidic.
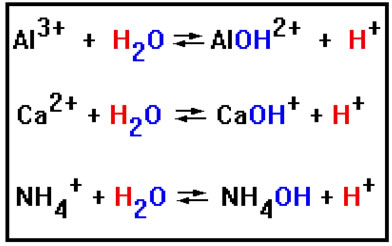 |
We have shown each positive ion bonding to only one hydroxide ion, even though some, such as the Al3+ and Ca2+ could bond to two or three. In the same way, a negative ion like SO42- could attract two H+ ions, but in the hydrolysis reaction we wrote, we showed it bonded to only one. To predict what actually happens requires some calculations. It will be sufficient for our purposes to show only a single H+ or OH- bonded to each ion. |
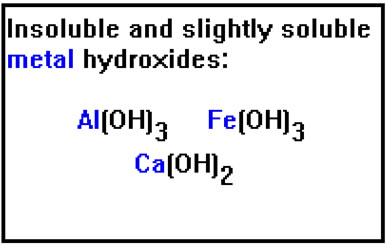
Just as the negative ions that cause basic hydrolysis come from weak acids, the positive ions that cause acid hydrolysis come from weak bases. You may recall that the list of strong bases included NaOH and KOH. RbOH and CsOH are also strong bases. LiOH is the only Group IA metal hydroxide that is not. The metal hydroxides in this list are weak bases because they do not dissolve well in water. They are either insoluble or only slightly soluble. |
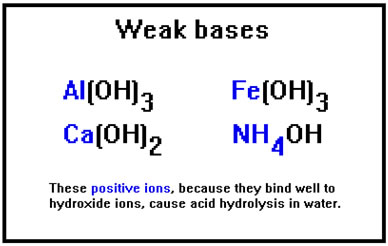 |
In general, only the Group IA metals (except Li) form highly soluble hydroxides and thus, strong bases. Ba(OH)2, Sr(OH)2, and AuOH are slightly soluble.
|
 |
NH4OH is also a weak base. However, it can dissolve in water and so is very soluble. It is the one weak base we will encounter that is. The ammonium ion forms a weakly basic hydroxide because, like most positive metal ions, it has a relatively strong attraction for hydroxide ions. This is the property that results in acid hydrolysis. Unlike the metals, its undissociated hydroxide dissolves in aqueous solution because it can form many hydrogen bonds with water. |
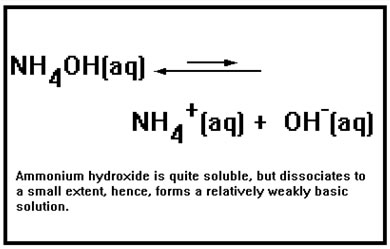 |
All of the positive ions that come from weak bases have a strong attraction for OH-. Therefore, when they dissolve in water, they induce hydrolysis by binding to the OH- ion in water and releasing the H+ ions into solution, turning the solution acidic. The stronger the attraction the positive ion has for hydroxide, the more acidic the resulting solution will be. The acidity of the solution also depends on the concentration of the ions. We will only be interested in being able to say whether the solution becones acidic or not. |
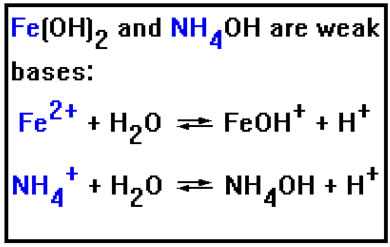 |
Positive ions that come from strong bases have a very weak attraction for OH- ions and thus do not cause hydrolysis to occur.
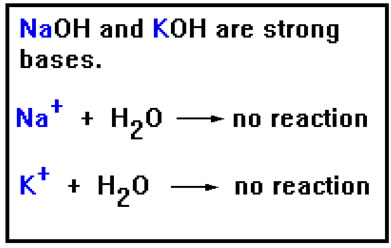 |
These, of course, are the positive ions associated with NaOH, KOH, RbOH, and CsOH. Since Ba(OH)2, Sr(OH)2, and AuOH are slightly soluble, the Ba2+, Sr2+, and Au+ ions do not cause appreciable hydrolysis as long as their concentration is low. |
Ions that cause acidic hydrolysis can be added to a solution in the form of ionic compounds, usually as nitrates or chlorates, but sometimes as chlordies, bromides, iodides, or sulfates, depending on the solubility of these compounds. All nitrate, acetate, and chlorate salts are soluble. Chlorides, bromides, iodides, and sulfates can be soluble or insoluble, depending on which positive ion they are combined with.
|
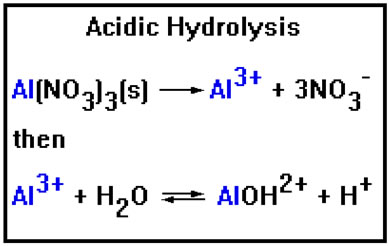 |
To determine whether an ionic compound will cause acidic hydrolysis, identify the positive ion, then the hydroxide compound it will form. If this compound is a strong base, no hydrolysis will occur, but if it is a weak base, then an acidic hydrolysis reaction will take place.
Choose the compounds that you think will cause an acidic hydrolysis reaction to occur when they are dissolved in water.
|
Once you have completed this exercise, see if you can write the hydrolysis reactions that will occur. Here is an example. The positive ion in AgNO3 is Ag+. This forms AgOH, an insoluble hydroxide, thus Ag+ will cause an acidic hydrolysis reaction:
|
Here are the answers you should have determined: |
|
|
Ag+ + H2O |
|
NH4+ + H2O |
|
Fe3+ + H2O |
An ionic compound can consist of both a positive ion that will cause acidic hydrolysis and a negative ion that will cause basic hydrolysis.
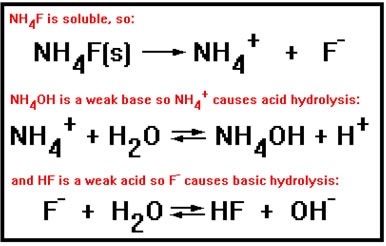 |
There are actually not too many examples of common ionic compounds that are both soluble and consist of a weak acid combined with a weak base. The ammonium compounds are the most plentiful. Most of the rest are only slightly soluble if they are soluble at all.
|
Four ionic compounds are listed in Exercise 11 in your workbook. Predict whether each of these will make an acidic, a basic, or a neutral solution. When you go in to the lab this week, make solutions from each of these salts and test your predictions.
In the next sections of the lesson, we’ll take up one of the most important principles in the study of chemical equilibrium, LeChatelier’s principle, and then we’ll see how it applies to solutions called buffers. Buffer solutions are vital to the survival of living things – they protect the sensitive enzyme systems in living tissue from changes in pH caused by external acids and bases.