Lesson 9: Lewis Structures
Exercise 5 in your workbook lists six molecules for which we will determine the shape based on their Lewis structures. To begin this section, work out the Lewis structures of the six compounds shown.

Here is a simple procedure for drawing a Lewis structure: (or you can review the appropriate section in Lesson 8)
- Arrange the atoms around a central atom – either the atom there is only one of or the largest atom. In molecules of H, O, and a third element, the third element will be the central atom.
- Count the total number of valence electrons.
- Place valence electrons around the outer atoms until the octet rule is satisfied.
- Place any remaining electrons around the central atom
- If the central atom does not have eight valence electrons around it, move a lone pair on one of the outer atoms to become a second shared pair with the central atom. Do this as many times as you need to in order to give the central atom 8 electrons.
We focus on the central atom in each Lewis structure. In each case, to specify the shape of the molecule we want to describe the geometric arrangement of the atoms that are bonded to the central atom. This arrangement is what we call the molecule’s shape.
Larger molecules typically have more than one “central” atom, that is, an atom that is bonded to two or more other atoms. In these cases, we describe the “shape” of the molecule by describing the arrangement of atoms around each of these central atoms individually.
Ex. 5 Answers: |
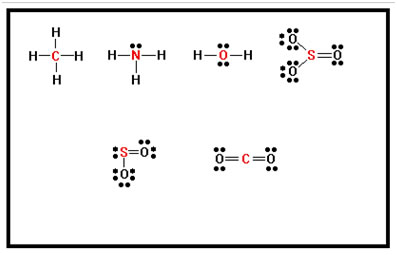 |
The arrangement of atoms around the central atom is, in turn, determined by the arrangement of the groups of electrons around the central atom. |
|
A group of electrons can be an unshared pair of electrons, a single bond, a double bond, or a triple bond. All electrons shared between two atoms are considered one group:
|
 |
In each case the electrons in a group move together around the central atom, getting as far as they can from the other groups of electrons around the central atom. |
|
Count the number of groups of electrons around the central atom for the six molecules we are working with and enter the numbers below the corresponding Lewis structures in Ex. 5 in your workbook.
Remember:
Each lone pair is one group; Each single bond is one group; Each double bond is one group; Each triple bond is one group. We are interested only in the number of groups that are around the central atom.
Answers: |
 |
Because electrons repel each other, the groups of electrons around the central atom get as far away from each other as possible. This is what determines the arrangement of the groups of electrons. The central atom is a sphere, so we want to know what arrangement of two, three, and four groups of electrons gets them as far apart as possible on the surface of a sphere.
The electrons within a double bond group or a triple bond also repel each other, but they are held together as a group by their mutual attraction to the nuclei of the two atoms sharing them.
You can get a good visual picture of where the groups of electrons go by getting four partially inflated balloons. If you hold two, three, or four balloons with their openings together between your fingers, the balloons will, like groups of electrons, get as far from each other as they can. The arrangement of balloons will mimic the arrangement of two, three, or four groups of electrons around a central atom.
When there are two groups of electrons, they move to exactly opposite sides of the central atom. Because this puts the two groups of electrons and the central atom in a straight line, it is called a linear arrangement of the groups of electrons. The two groups of electrons can be a lone pair and a triple bond or two double bonds. These are the only two ways to get eight electrons around a central atom in only two groups. |
 |
When there are three groups of electrons around a central atom, the farthest they can get from each other is to move towards the corners of a flat triangle. This arrangement is called either trigonal planar, flat triangular, or planar triangular. The angle between adjacent groups of electrons will be about 120o. All three groups of electrons and the central atom will lie in the same plane – that is, they will lie flat. The three groups of electrons could be: Note that all of these combinations involve exactly one double bond. |
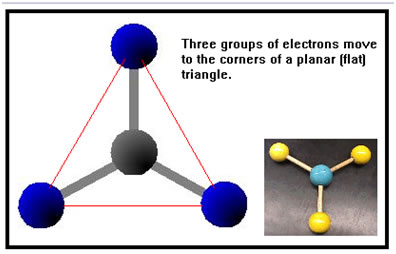 |
When there are four groups of electrons around a central atom, they maximize the distance between them by moving to the corners of a tetrahedron. This arrangement is called tetrahedral. The four groups of electrons could be: Notice that four groups of electrons are associated with central atoms that have no double or triple bonds. |
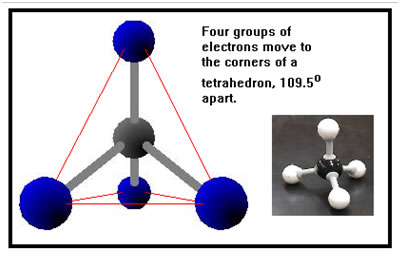 |
Practice this step by entering in your workbook the name for the arrangement of groups of electrons around the central atom in each of the six compounds.
The arrangements are quite regular:
Four groups are always arranged tetrahedrally; three groups are always arranged in a planar triangle; two groups are always arranged linearly.
Large central atoms can violate the octet rule and have 5 or 6 groups of electrons. We will not deal with these compounds in this course.
Answers: |
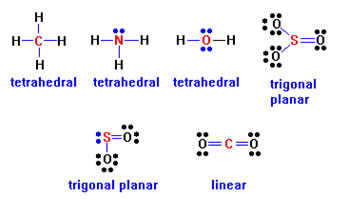 |