Lesson 9: Molecular Shapes
The last step in this procedure is based on the fact that the shape of the molecule describes the arrangement of the atoms that are attached to the central atom, not the arrangement of the groups of electrons. The shape of a molecule is affected by the arrangement of electrons, but does not describe that arrangement.
The “shape” of a molecule is how the outside atoms are arranged around the central atom. The lone pairs of electrons affect this arrangement, but are not part of it. It’s important to distinguish between the arrangement of groups of electrons and the arrangement of atoms around the central atom. If there are no lone pairs of electrons, these two arrangements will be the same, but if there are lone pairs the arrangement of groups will be different than the arrangement of atoms.
When Arrangement = Shape | Bent Shape | Triangular Pyramidal Shape | Linear Shape | Practice
When Arrangement = Shape
If the number of atoms is the same as the number of groups of electrons – in other words, if there are no unshared pairs of electrons around the central atom – the shape of the molecule will be the same as the arrangement of groups of electrons: linear, trigonal planar, or tetrahedral.

It is as if lone pairs of electrons are invisible – ghosts that affect the shape of the molecule but are not part of it. We can only “see” the atoms bonded to the central atom. If there are lone pairs, the atoms bonded to the central atom are pushed apart by the invisible lone pairs and the shape of the molecule will be different than if there were no lone pairs.
We won’t use this fact until the next page in the lesson, but notice that these three shapes – linear, trigonal planar, and tetrahedral – are all symmetric shapes, the atoms are arranged evenly around the central atom. The symmetry of these shapes will help determine whether the molecule is polar or nonpolar, and consequently what kind of intermolecular bonds determine its melting point, boiling point, and other physical properties.
Bent Shape
There are two situations in which a molecule's shape is "bent" or "angular." Both involve molecules with lone pairs on the central atom.
When there are three groups of electrons around the central atom, but only two atoms, these atoms are said to be in an angular or bent arrangement. Notice that the lone pair of electrons is pushing the two bonds down, causing them to be closer together than if there were no lone pairs. This angular – or bent – arrangement of the bonds gives the molecule its shape. The angle between the bonds is approximately 120o. The second figure shows what the molecule looks like the when the “invisible” lone pair is not shown.
|
 |
And when there are four groups of electrons around the central atom, but only two atoms, the molecule is said to have an angular or bent shape. This shape is similar to the angular shape of a molecule with three groups of electrons and two lone pairs. The major difference is that in this case the angle between the two bonds is smaller (about 109.5o) than it was with three groups of electrons and one lone pairs (about 120o). Two lone pairs of electrons push the bonds farther down than one lone pair does. |
 |
Triangular Pyramidal Shape
There is only one situation in which the shape of a molecule is a triangular pyramid. It, too, involves a molecule with a lone pair on the central atom.
When there are four groups of electrons around the central atom, but only three atoms, the molecule is said to have a trigonal pyramid or triangular pyramid shape. Again, the lone pair is pushing the bonds down. The shape of the molecule is the bottom half of the tetrahedron, called a trigonal pyramid (or triangular pyramid). The difference between this shape and a tetrahedral shape (both of which can be imagined inside a pyramid with a triangle for a base) is that in a tetrahedral shape the central atom is in the middle of the pyramid, while in a trigonal pyramidal shape, the central atom is at the top of the pyramid. |
 |
Linear Shape
No matter how many groups of electrons there are, when there is only one atom bonded to the central atom, the two atoms are in a linear arrangement. The two atoms are the two points that define a line.

This occurs when there are two groups of electrons and one lone pair (one bond), three groups of electrons and two lone pairs (one bond) and four groups of electrons and three lone pairs (one bond).
Practice
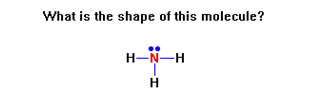
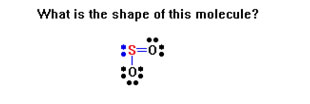
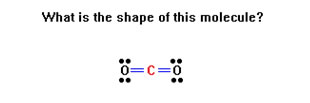
To review, for each Lewis structure determine the shape of the molecule. Remember, you are being asked for the shape of the molecule, not the arrangement of the groups of electrons.
Remember to determine first the arrangement of groups of electrons – either linear (2 groups), trigonal planar (3 groups), or tetrahedral (4 groups).
If there are no lone pairs, these will also be the shapes of the molecule.
One lone pair turns a trigonal planar arrangement into a bent molecule and a tetrahedral arrangements into a trigonal pyramidal molecule.
Two lone pairs turn a trigonal planar arrangement into a linear molecule and a tetrahedral arrangement into a bent molecule.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
To practice this procedure, complete Exercise 6 in your workbook.
Here are the answers: CH3Cl tetrahedral; CH2Cl2 tetrahedral; HCN linear; CH2O trigonal planar; O3 bent; BH3 trigonal planar (in this molecule there are only six electrons around the central atom); PCl3 trigonal pyramidal.
In the next page, we'll determine a molecule's polarity based on its shape.