Lesson 9: Miscibility
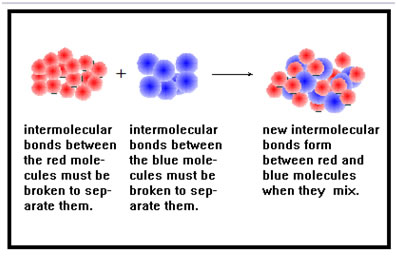
Intermolecular bond types also determine whether two chemicals are miscible, that is, whether they can be mixed together to form a homogeneous solution. But here, the key is whether the two materials use similar intermolecular bond types.
If two chemicals have dissimilar bond types, the energy required to break the bonds between molecules that use the stronger intermolecular bond type will be more than the energy released when new bonds form between the two different kinds of molecules. The net loss of energy is what prevents the two from mixing.

When two chemicals mix, the bonds holding the molecules of each chemical together must break, and new bonds form between the two different kinds of molecules. For this to happen, the two chemicals must have compatible intermolecular bond types.
The more nearly equal in strength the two intermolecular bond types are, the greater will be the miscibility of the two chemicals. Usually there is a limit to how much of one chemical can be mixed with another, but in some cases, such as with CH3OH and H2O, there is no limit and any amount of one is miscible in any amount of the other.
The next table shows the result of mixing a variety of different chemicals. Examine the pictures on each screen and fill in the appropriate section of the tables in Exercise 13. (This is also set up for you to observe in the lab.)
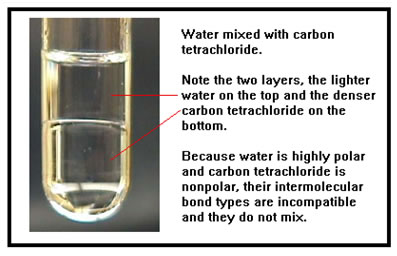
We begin with water (a polar chemical) mixed with carbon tetrachloride (a nonpolar chemical). The bright line about half way up the liquid is where the water and the carbon tetrachloride meet. This surface reflects light just like the surface of any liquid, making it easier to see. Careful measurements detect practically none of either liquid mixed in with the other. |
 |
In this photo, iodine, I2, is mixed with both water and carbon tetrachloride. How much iodine dissolves in each liquid is reflected in how deep is the color of the resulting solution. Clearly there is a great deal of undissolved iodine in the water. The solution of iodine in carbon tetrachloride is so dark that it is difficult to see whether any solid iodine remains. None does. All of the iodine has dissolved in the carbon tetrachloride. |
 |
In these photos, sugar is mixed with water and with carbon tetrachloride. Sugar contains many hydrogen atoms bonded to oxygen atoms and therefore is very polar. Sugar is just as soluble in water as iodine is in carbon tetrachloride. Common table sugar consists of two rings of carbon atoms. On each carbon atom except one there is an oxygen which is bonded, in turn, to a hydrogen. Each sugar molecule contains eight O-H bonds. No wonder it is so soluble in water. |
 |
Here is a photo of ethanol, CH3CH2OH, in both water and carbon tetrachloride. Ethanol contains both a nonpolar group, the CH3CH2 or hydrocarbon end, and a polar group, the OH end. Ethanol, also called ethyl alcohol, is the alcohol in alcoholic beverages. It’s dual nature – the molecule has both polar and nonpolar regions, means it dissolves in both the blood and in the body’s fatty tissue, which is one reason it is able to reach the brain. The other reason is its small size. |
 |
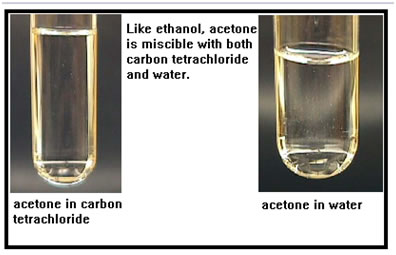
Acetone is similar to ethanol, it has both a polar and a nonpolar section. This is acetone mixed with water and with carbon tetrachloride. Ethanol is mildly toxic. Acetone is much more so. Acetone is sometimes produced in the body when the diet is heavy in protein and low in carbohydrates. When this happens, the body goes into a condition known as ketosis and the telltale odor of acetone can be detected on the breath. Ketosis, if it is not treated, can lead to death. |
 |
What would you predict will be the result of mixing iodine, a nonpolar molecule, first with acetone, and then with ethanol? You can see the result of mixing these two combinations in the photo. Describe what you see in part c. of Exercise 13. You should have predicted that iodine would be miscible with both ethanol and acetone. Both of these chemicals have a nonpolar portion of their molecule that is sufficient to allow them to mix with carbon tetrachloride, therefore one would expect that they would be miscible with other nonpolar molecules, such as iodine. |
 |
Summarize what happens when iodine and sugar are mixed with water and carbon tetrachloride by completing the table in part d of Exercise 13. If you do this correctly, you will see that when two chemicals do mix, the bond types that form are the same as the bond types that are broken.
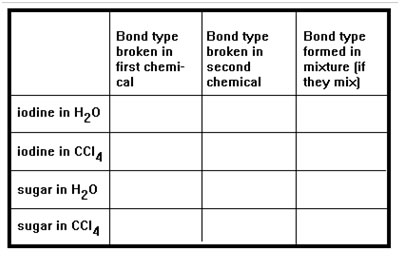
The bonds that break when a chemical begins to mix with another are just the intermolecular bonds that determine its melting and boiling points. You should have no trouble predicting the type of bond this is for the four chemicals in the chart. (If you have trouble with this, get help from an instructor.)
Normally, network materials do not mix with other chemicals because the network bonds are just too strong. The exception to this is that some ionic materials will dissolve in some polar liquids – most commonly water. Ordinary salt, for example, will dissolve easily in water.
The strongest ionic bonds form between ions of high charge. The ionic solids that tend to dissolve most readily are those in which the ions have +1 and -1 charges, like NaCl. The higher the charges on the ions, the less likely an ionic solid is to dissolve.
Among liquids, water is far and away the most polar. It is the only liquid in which a large number of ionic solids will dissolve.
 |
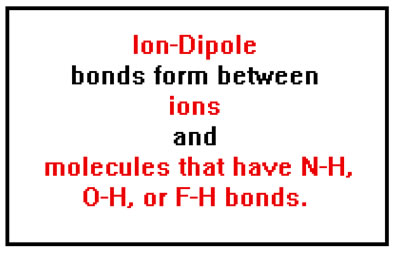 |
When an ionic compound dissolves in a very polar liquid like water, the ionic bonds are broken and the ions separate. Some of the hydrogen bonds between water molecules also break and the water molecules separate to make room for the dissolving ions. Finally a new type of bond we have not seen before forms. This bond is called an ion-dipole bond.
Ion-dipole bonds are not as strong as ionic bonds. Part of this is made up for by the fact that a large number of ion-dipole bonds can be formed with each ion. Many water molecules cluster around each sodium and chloride ion in solution. This larger number of ion-dipole bonds helps make up for the fact that they are weaker than the ionic bonds they replace.
Ion dipole bonds form because the negative ends of many water molecules are attracted to each positive ion, while the positive ends of many other water molecules are attracted to each negative ion. |
 |
Ion-dipole bonds are stronger when the charged species are smaller and the charge is more concentrated. This is another reason why water is such a good solvent for ionic compounds. The negative charge is concentrated on an oxygen atom, which is relatively small, and the positive charge is on a hydrogen atom, which is the smallest atom in the periodic table.