Lesson 9: Network & Molecular Materials
Begin this lesson by completing the table in Exercise 1 in your workbook as a way to review what you learned in Lessons 7 and 8. If you need help, you can scroll down the page to see the answers. But see if you can complete the table without assistance. Check your answers when you are done.
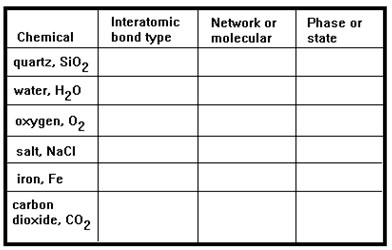
You determine the interatomic bond type by noting whether the material includes just metals, just nonmetals, or both. The interatomic bond type will be metallic, covalent, or ionic, respectively.
All ionic and metallic materials are network materials. Most covalent materials are not, though you were asked to remember a few covalent materials that are network materials: diamondand graphite (both pure carbon), pure silicon, and quartz, SiO2.
You should be able to fill in the third column, the phase, or state of the material from your own experience.
Study the completed table. The phase at room temperature does not seem to be determined by the bond type. Quartz, water, oxygen, and carbon dioxide all exhibit covalent bonding, yet one is a solid, one is a liquid, and two are gases. In fact, all three of these bond types (covalent, ionic, and metallic) are very strong. Based on this alone, all six of these materials should be solids. |
|
The stronger the bonds, the higher the melting point and boiling point. A material held together by very strong bonds should require a great deal of energy – means high temperature – to break apart into the liquid and gas phases, and so should be a solid at ordinary temperatures. The fact that water is a liquid and oxygen and carbon dioxide are gases at room temperature suggests that it is not the covalent bonds that are being broken when these materials melt, vaporize, or, in the case of carbon dioxide, sublimate. |
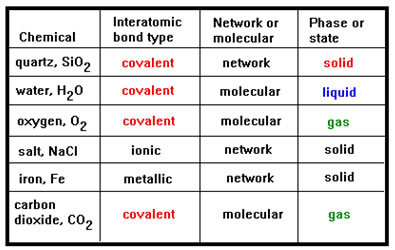 |
One clue to this apparent contradiction is that only the network materials have the high melting and boiling points that we would expect if strong covalent, ionic, or metallic bonds were holding the atoms and molecules together.
The solids at room temperature are quartz, salt, and iron. Quartz is a network covalent material, salt is a network ionic material, and iron is a network metallic material. In general, for atoms of the same size, covalent bonds are the strongest, ionic bonds are the next strongest, and metallic bonds are the weakest. All of these bonds, however, are classified as strong bonds, which is why all of these materials are solids at room temperature.
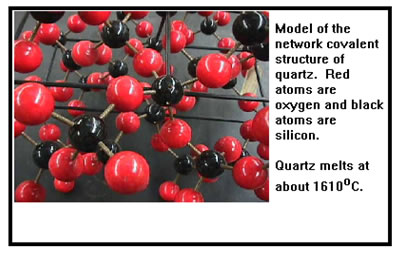
In network materials the entire sample of material is held together by the strong interatomic bonds you identified in the table. This is illustrated by the model of a quartz crystal shown. To melt quartz, these strong covalent bonds must be broken which requires a very large amount of energy. Quartz melts at about 1610 oC, salt (sodium chloride) melts at about 800 oC, and aluminum, a third period metal comparable in size to sodium, chlorine, and silicon, melts at about 660 oC. Iron, a fourth period metal, melts at about 1500 oC. An ionic compound containing a fourth period metal is calcium oxide, which melts at about 2600 oC. Diamond, the strongest covalent network material known, can be melted in a vaccum at about 3550 oC. |
 |
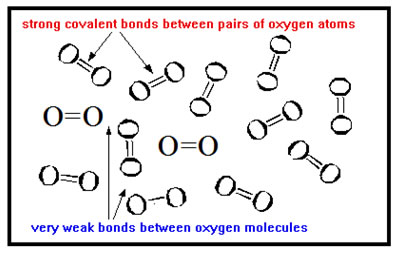
But in molecular materials, the strong interatomic covalent bonds hold together only the atoms within each molecule. These strong bonds do not exist between molecules.
Compare the bonding of a dozen iron atoms to the bonding of a dozen oxygen atoms. The strong metallic bonds that hold iron atoms together hold all twelve atoms together. The strong covalent bonds that hold oxygen atoms together bond pairs of atoms into six separate molecules. Each oxygen atom is strongly bonding to only one other oxygen atom. Each molecule is essentially unattached to any other molecule, which is why oxygen is a gas. |
 |
Oxygen forms a liquid at -183 oC, carbon dioxide forms a solid at -78.5 oC, and water is a liquid at room temperature. Clearly, there must be some form of bonding between molecules, otherwise they could never be found in states other than a gas. The strength of these bonds must be much lower than covalent, ionic, or metallic bonds and it also must vary from one molecule to another.
Even though bonds between molecules are weak, they are very important. Not only do they influence melting and boiling points, they also help determine whether chemicals will or will not mix with one another. They are important in biochemistry also. These are the bonds that hold together the two strands of DNA. They also help determine the shape and function of proteins. On a more mundane level, these bonds are what let soap do its work cleaning things.
Bonds between molecules are called intermolecular bonds. There are three different types of intermolecular bonds: van der Waal’s bonds, dipole-dipole bonds, and hydrogen bonds. We will describe these in some detail in the next section.
Intermolecular bonds are the bonds between molecules. Interatomic bonds are bonds between atoms. In a network material, all of the bonds are interatomic bonds because there are no discrete molecules. In fact, what distinguishes a network material from a molecular one is that in a molecular material there is a difference in the type and strength of the bonds within a single molecule and between molecules. Without this difference, it would not be possible to tell where one molecule ended and another began.