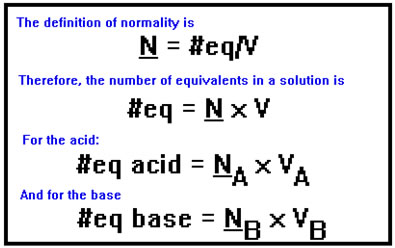Lesson 6: Titration Calculations
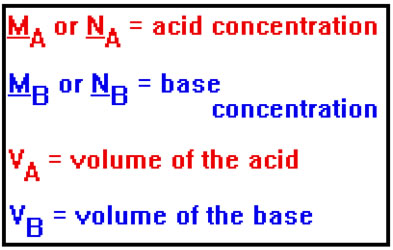
When you complete a titration, you will have three pieces of data: the volumes of the acid solution and the base solution required to reach the endpoint and the concentration of one of those two solutions.
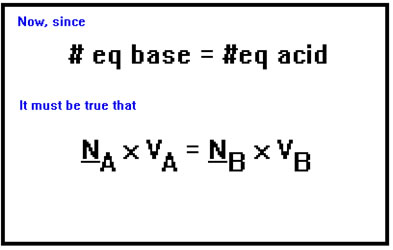
The calculations are easiest when you use normality for the concentration, because normality is based on equivalents and one equivalent of any acid exactly neutralizes one equivalent of any base. Therefore, at the endpoint, the number of equivalents of acid must be the same as the number of equivalents of base.

Recall that an equivalent is the amount of acid (or base) that delivers one mole of H+ (or OH-) ions. If the number of equivalents of acid is the same as the number of equivalents of base, then the number of H+ ions must be the same as the number of OH- ions, and the two will just exactly neutralize each other.
To use normality in your calculations, you may have to first convert a concentration in moles per liter to normality. To do this, simply multiply the molarity of the solution by the number of equivalents per mole of the acid or base.
Based on the definition of normality, the number of equivalents in a given volume of solution is just that volume times the normality of the solution.
|
The same equations can be used for moles and molarity. The number of moles, n, is just equal to the volume of a solution times its molarity. However, since one mole of acid can contain 1, 2, 3, or more moles of H+ ions, and a base can contain 1, 2, 3, or more moles of OH- ions, the number of moles of acid is not necessarily the same as the number of moles of the base at the endpoint.
|
|
Since the number of equivalents of acid and base are the same, the normality times the volume of acid must be equal to the normality times the volume of the base. To use molarity, we would have to take into account the relative numbers of moles of acid and base that contain equal amounts of H+ and OH- ions. For example, if we were titrating H2SO4 with NaOH, the appropriate equation using molarity would be 2 x MA x VA = MB x VB because the number of moles of NaOH would have to be twice the number of moles of H2SO4.
|
|
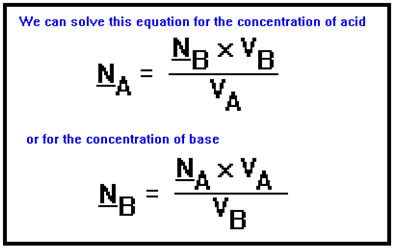
Since you know both volumes and one of the concentrations, it’s an easy matter to solve for and compute the concentration you don’t know. In these equations, notice that one volume is divided by the other. This means that it is not necessary to convert the volumes from milliliters to liters before performing the calculations. It is necessary, however, to have the two volumes expressed in the same units. Those units, no matter what they are, will then cancel. |
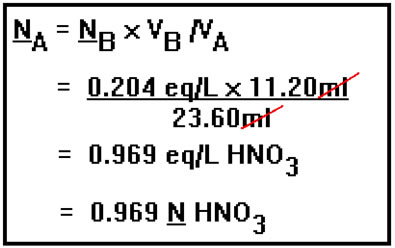
Suppose, for example, that the titration of 23.60 mL of HNO3 with 0.204 N NaOH requires 11.20 mL of the base. To find the concentration of the acid, we solve the titration equation for NA, plug in the values for the two volumes and the normality of the base, and perform the arithmetic. Even though the concentration of base is expressed in equivalents per liter, it was not necessary to change the volumes from milliliters to liters. The units of the volumes, mL, canceled.
|
 |
Exercise 16 in your workbook has several examples of titration problems for you to try. Answers can be found below.
In part d. of Exercise 16, note that the concentrations are given as molarities, not normalities, not normalities. The balanced equation for the neutralization reaction shows that it takes 3 moles of Ba(OH)2 to neutralize 2 moles of H3PO4.
2 x (3 mol Ba(OH)2) = 3 x (2 mol H3PO4)
Or
2 x nB = 3 x nA
Alternatively, you could convert the concentrations from molarities to normalities and solve the problems in the same way you did the previous ones.
Answers to Exercise 16:
16. a. 0.120 N
b. 0.41 N or 0.21 M
c. 0.020 N or 0.010 M
d. 0.061 N or 0.030 M (The conc. of H3PO4 is given in M, but you need to use N to do
the calculation correctly.)