Lesson 5: Radiation
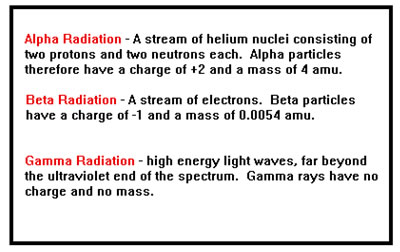
By 1909 Ernest Rutherford and others had established the existence and nature of three kinds of radiation: alpha rays, beta rays, and gamma rays. Alpha rays consisted of helium atoms with a charge of +2 and a mass four times that of protons. Beta rays were just electrons, and gamma rays were a form of light.
The first type of radiation discovered was, of course, the cathode rays. Then, in 1895, Roentgen discovered xrays, which were produced by the cathode rays hitting the anode of a CRT.
In 1896, Henri Bequerel observed xrays occurring naturally in sample of uranium he was working with.
In 1897, J. J. Thompson found that some natural radiation consisted of negative particles. By 1899, Rutherford had confirmed Thompson’s discovery and had also identified a third form of positively charged radiation. He called these alpha rays. He called the negative radiation beta rays.
Finally, in 1900, Villard identified a third form of natural radiation, which were dubbed gamma rays. These were neutral and had no mass at all.
In 1903, Ramsey and Soddy showed that in the process of giving off radiation, uranium formed helium gas.
By 1909, Rutherford was able to proved that the helium was produced when the particles alpha radiation gained two electrons.
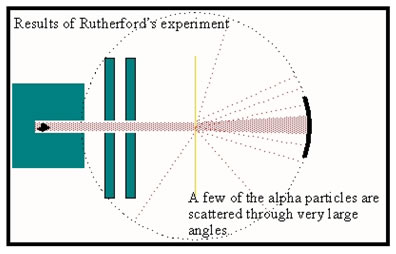
To test J.J. Thomson’s plum pudding model, Rutherford directed a beam of alpha particles at a thin leaf of gold foil. If Thomson’s model were correct, the alpha particles should pass right through the foil. The much lighter protons and electrons would not be heavy enough to deflect the more massive and fast moving alpha particles.
There would, of course, be some scattering, but all of the alpha particles should emerge from the gold foil along a path only slightly wider than the original beam, as shown in the diagram.
The detector, mounted on a track indicated by the dotted circle, should show many alpha particle when placed directly behind the gold foil, but as it moves around the circle out of the direct path of the beam, the rate of detection should drop to zero not very far to either side – that is, if the plum pudding model is correct. |
 |
When the experiment was conducted, most of the alpha particles did, in fact, pass directly through the foil. But a few were deflected to the side and, much to Rutherford’s surprised, every so often an alpha particle would bounce off the foil completely.
|
 |
Rutherford repeated his experiment many times with many different kinds of metal foils and the result was always the same. Rutherford became convinced that the “only logical structure” for the atom could not be the correct one.
Rutherford proposed that the atom was mostly empty space occupied by the electrons, thus most of the alpha particles passed through the foil unperturbed. The protons were concentrated at the center of the atom – in a nucleus. The occasional alpha particle that came near the nucleus was deflected. A rare direct hit would cause the alpha particle to bounce off the foil. Because the nucleus, like the alpha particles, was positively charged, an alpha particle only had to come near it to be deflected. The repulsion between the like charges was enough to change the path of the alpha particles.
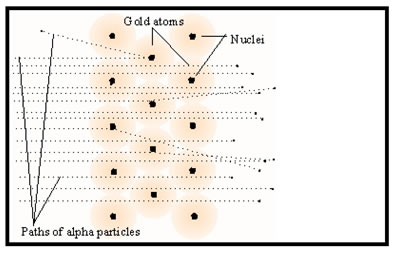
Neutrons had not yet been discovered, so Rutherford did not include them among the particles he thought were to be found in the nucleus.
Studies of radioactive decay lead to the eventual discovery of isotopes when it was found that there were more kinds of atoms than there were elements. It was not until the 1930’s, however, that the existence of neutrons was proved, and isotopes were shown to be atoms with the same number of protons, but different numbers of neutrons.
When thorium emitted radiation, it changed into a different element. This, in turn, gave off more radiation and became yet another element, and the process continued until eventually an atom of lead was formed and no more changes occurred.
Only seven elements exist between thorium and lead, but ten different atoms were found to be part of the sequence in the radioactive decay of thorium to lead. The extra atoms could only be accounted for if there were more than one form of at least some of the elements. It has turned out that every element has several isotopes.
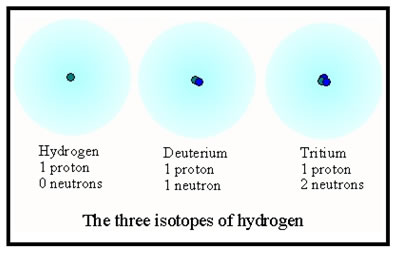 |
Of the three isotopes shown, the first accounts for 99.985% of hydrogen atoms. Deuterium accounts for 0.015%, virtually all the rest. Fewer than one atom in 100,000 is tritium, the radioactive form of hydrogen. |
In addition to radioactive decay, Rutherford also discovered he could create larger atoms from smaller ones by directing alpha particles at their nuclei. When an alpha particle stuck, the atomic number would increase by two and a new element would form. This process is called nuclear fusion. The fusion process shown here is simpler, it is the one that takes place in a hydrogen bomb.
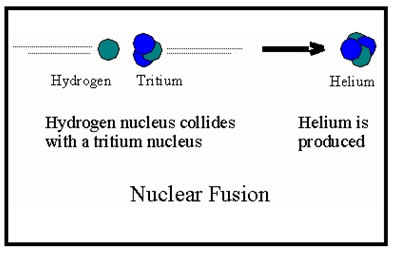
When a nucleus loses an alpha particle and its atomic number decreases, the process is called nuclear fission.
In the 1930’s Irene Joliot-Curie, daughter of Marie and Pierre Curie, produced artificial radiation. In 1934, Enrico Fermi began experiments in which he bombarded nuclei with neutrons instead of alpha particles. The process of controlled nuclear fission was not achieved until the 1940’s, however, and controlled fusion was finally achieved in the 1950’s.
The real nature of atoms turned out to be quite different and much more complex than John Dalton, who first proposed their existence in the early 1800’s, ever imagined.