Lesson 5: Wave Mechanics
Bohr's model was the first to propose that electrons were found in specific energy levels, which he called orbits; with his model, he was able to correctly predict the emission spectrum of hydrogen atoms (H), helium ions (He+), and lithium ions (Li2+) -- each of these has only one electron. However, his model could not correctly predict the spectra of atoms or ions with more than one electron.
Wave mechanics is a sophisticated mathematical treatment of atoms. It kept Bohr's idea that only certain energies are allowed, but not his notion that the electrons are restricted to certain distances from the nucleus. For Bohr's orbits it substituted "orbitals."
Wave mechanics (and quantum mechanics, which is closely related) were developed in the first part of this century to explain the properties of atoms and molecules. The physical laws known as Newtonian physics that describe the motion and interactions of ordinary objects do not work for things as small as atoms. Odd as the laws of quantum mechanics are, they are identical to Newtonian physics when they are applied to objects of ordinary size and mass.
Orbitals | Energy Levels | Electron Organization
Orbitals
An orbital is a mathematically-defined region in the space around the nucleus in which there is a high probability of finding the electron. Like Bohr’s orbits, there are an infinite number of orbitals, but they can only have certain allowed energies. Different orbitals have different three-dimensional shapes as well.
An orbital is not a physical object any more than an orbit is. When we speak of an electron being “in” a particular orbital, we mean that the electron has the potential energy, and is found in the region, defined by that orbital. In a sense, an orbital is a visual and mathematical means of describing an energy state that an electron may have in an atom. The details of the energy state are influenced by the charge on the nucleus and by the presence of other electrons. For that reason, no two atoms or ions have exactly identical orbitals. However, they are similar enough that we use a common set of names to designate them.

Energy Levels
The orbitals in an atom are grouped into energy levels, called “main energy levels,” which are labeled 1 (the first main energy level), 2 (the second main energy level), and so on. The higher the energy level, the larger it is, the higher the energy of the electrons it contains, and the farther they are from the nucleus. |
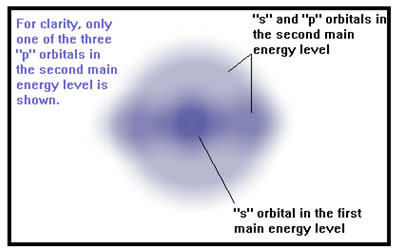 |
(When we say that electrons in higher main energy levels have greater energy, we are talking about potential energy.
The situation is similar to a flower pot sitting on your front porch stoop – a low potential energy state – compared to a flower pot on the upstairs balcony – the higher potential energy state. Neither flower pot is moving, so neither one has appreciable kinetic energy. But the flower pot on the upstairs balcony has greater potential to do damage if it's dropped to the ground than the one on the porch.)
Also, the higher the main energy level, the more orbitals it contains. The first main energy level has only one orbital, called and "s" orbital. The second main energy level also has an "s" orbital and three "p" orbitals as well. |
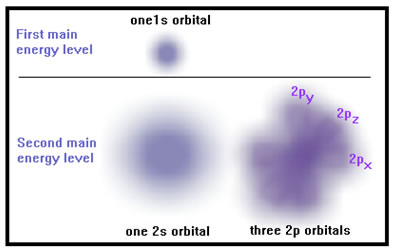 |
The electrons are attracted by the nucleus but repelled by each other. (Remember that opposite charges attract and like charges repel.) In the first main energy level, there is not much room and adding more than a couple electrons would result in electron-electron repulsions that are far larger than their attraction to the nucleus. In the second main energy level there is much more room, so more electrons can occupy it without being too repelled by each other. The shapes and orientations of the orbitals minimize electron-electron repulsion and maximize the attractive force from the nucleus.
Electron Organization
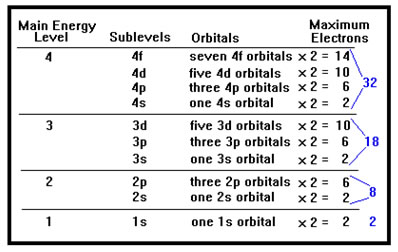
If you study the chart, you will notice that there is only one "s" orbital in each main energy level, that "p" orbitals always occur in groups of three, "d" orbitals in groups of five, and "f" orbitals in groups of seven. The pattern continues with later orbitals as well. Each letter group of orbitals within a main energy level is called a sublevel. Take some time to study the chart. Once you recognize the patterns, it will be much easier to remember all the names of the orbitals and the number of electrons each orbital, each sublevel, and each main energy level can hold. Each of the three "p" orbitals in a "p" sublevel has a separate designation, they are the px, the py, and the pz orbitals. We will not be too concerned with those names, but you will have to remember how many orbitals there are in each sublevel. |
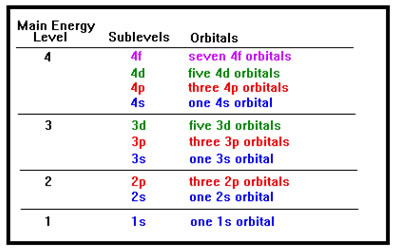 |
Each orbital can hold two electrons. For example, the three p orbitals in the second main energy level are collectively called the "2p sublevel" and this sublevel can contain up to six electrons, 2 in each of the three 2p orbitals. This is true for any orbital in any sublevel of any main energy level. One orbital can always hold exactly two electrons, whether it's a d orbital in the fifth main energy level or an s orbital in the first main energy level. It is the sublevels that can hold different numbers of electrons because they contain different numbers of orbitals. |
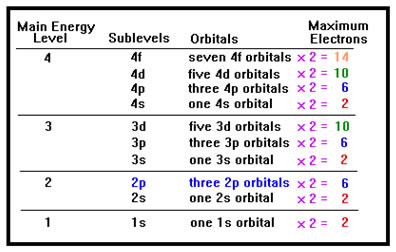 |
Since p orbitals always occur in groups of three, a p sublevel can always hold up to six electrons, no matter what main energy level it's in. Since d orbitals always occur in groups of five, a d sublevel can always hold up to ten electrons, two per d orbital in the sublevel. The distinction between main energy levels, sublevels, and orbitals is frequently confusing to students. The fact that the sublevels and the orbitals have the same names does not help. A 2p sublevel contains three 2p orbitals. A 3d sublevel contains five 3d orbitals. If you learn to look for the word "orbital" and "sublevel" after the designation, the patterns won't be so confusing – p sublevels can always hold 6 electrons because they always have three p orbitals. The d sublevels all have five d orbitals and can hold 10 electrons. The s sublevels always have a single s orbital and can hold only two electrons. |
 |
At this point you may be feeling a bit overwhelmed with all the details involved in the wave mechanical model of the atom. For now it will be enough if you understand some of the basics about the wave mechanical model and have a general idea of how this model differs from the Bohr model. (Example 17 summarizes the points you need to know about the wave mechanical model.) In the next lesson we will use the periodic table to see how its arrangement relates to the wave mechanical model of the atom.
Examples 18 and 19 are two ways of showing the arrangements of energy levels, sublevels and orbitals. Example 18 has the information grouped by main energy levels; Example 19 shows how the energy levels relate to (and sometimes overlap with) each other. In the last section of this lesson, "Electron Configuration," we'll spend a bit more time with the wave mechanical model and Example 19 as we look at how the electrons are arranged within the atom.